
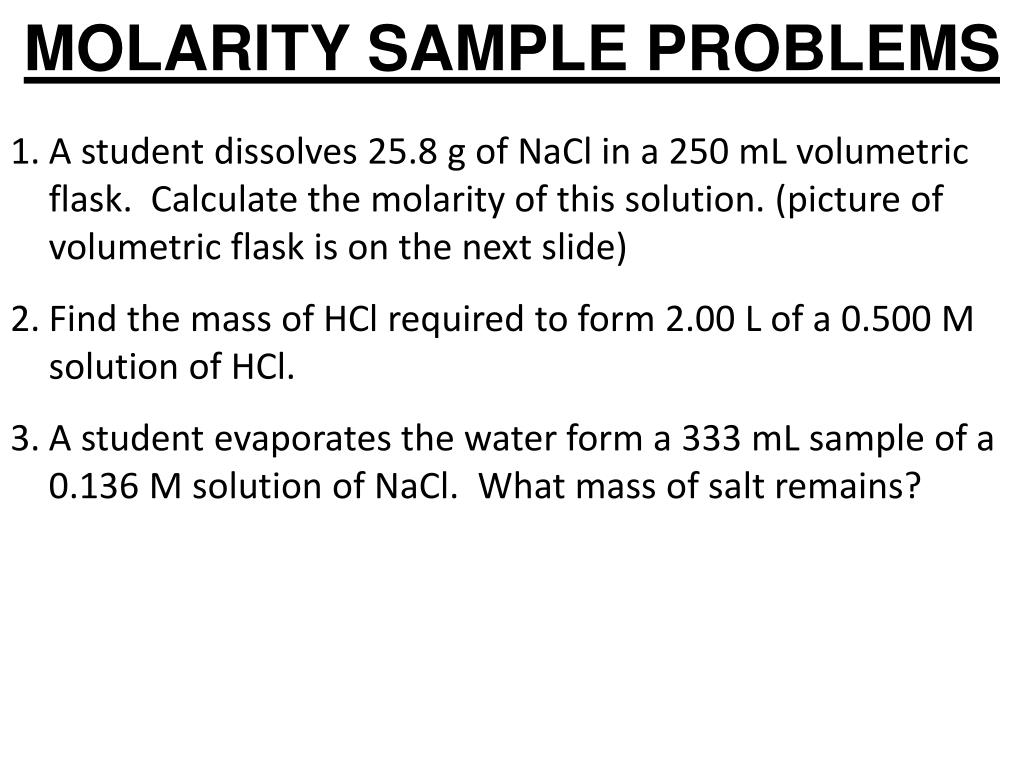
With lower densities (large volume at low pressure) the neglect of molecular size becomes less critical since the average distance between adjacent molecules becomes much larger relative to the size of the molecules themselves. The major issue with the idea gas law is that it neglects both molecular size and intermolecular attractions, therefore it is most accurate for monatomic gases at high temperatures and low pressures. If a real gas behaves sufficiently like an ideal gas the formula can be used as an approximation depending on the required margin of error. The equation for the ideal gas (PV=nRT) applies only to, well, an ideal gas. Understanding when the ideal gas formula applies and when it does not is a key prerequisite in making sure you use this ideal gas law calculator accordingly. The appropriate formula from the ones listed above is chosen automatically when you use this ideal gas law calculator. The combined gas law formula states that with a constant quantity of gas the gas pressure multiplied by its volume and divided by its temperature is also constant: Under these conditions, if two gases have the same volume, they must necessarily contain the same molecular quantities. With Gay-Lussac's law we have that for a constant volume and gas quantity the pressure of a gas divided by its temperature is a constant:Īvogadro's law states that if we have constant temperature and pressure the gas volume divided by the gas quantity is a constant. With Charles' law we have that for a constant pressure and gas quantity its volume divided by its temperature is constant: This means that under the same temperature, two gases with equal quantity of molecules and equal volume must also have the same pressure, as well as that two gases with equal quantity and pressure must have the same volume. With Boyle's law we have that for a constant temperature and gas quantity the pressure of a gas multiplied by its volume is also constant: The ideal gas formula was first stated by the French engineer and physicist Emile Clapeyron in 1834 based on four component formulas, discussed below.

T: the number of gas molecules times the Boltzman constant times the absolute temperature.A mole is the amount of substance which contains as many elementary entities as there are atoms in 12 g of carbon-12.Īnother way to express the right side of the equation is N
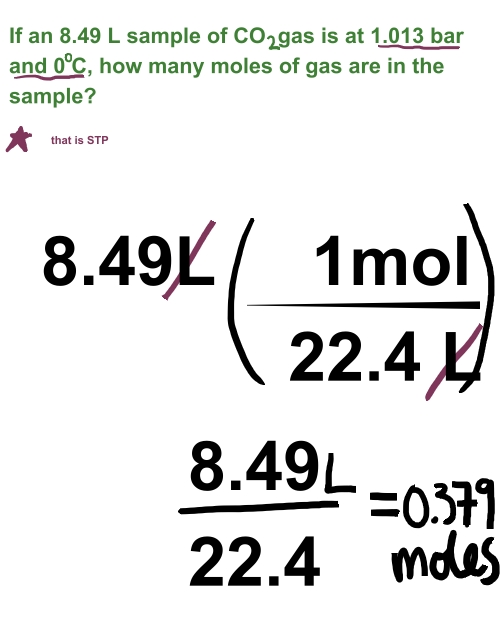
Due to this formula people would often refer to the above tool as a " PV nRT calculator". R is equivalent to the Boltzmann constant, but expressed in units of energy per temperature increment per mole (the pressure–volume product). Where P is the pressure in Pascals, V is the volume in m 3, n is the quantity in moles, T is the absolute temperature in Kelvins and finally R is the universal gas constant. The ideal gas law is the equation for the state of a hypothetical ideal gas. The gas law calculator uses a combination of several formulas for the behavior of gases which can be derived from four separate gas law formulas and result in the ideal gas formula shown below. Units supported for pressure are Pascals, kiloPascals, MegaPascals, GigaPascals, millibars, bars, atmospheres, millimeters of Hg liquid, millimeters of H 2O liquid, and pound-force per square inches (psi). The units supported for volume are: mm 3, cm 3, m 3, ml, L (litre), gallons, fluid ounces, cubic inches, cubic feet and cubic yards. It supports both imperial and metric units for volume and pressure and 5 different temperature scales: Kelvin, Celsius, Fahrenheit, Rankine and Reamur, both as input and as output. The calculator uses the combined gas law formula discussed below to perform the computations. Simply enter the three known measures to calculate the fourth. This is an ideal gas law calculator which incorporates the Boyle's law, Charles's law, Avogadro's law and Gay Lussac's law into one easy to use tool you can use as a:


 0 kommentar(er)
0 kommentar(er)
